Posted on January 12, 2023

The Asbestos Disease Awareness Organization (ADAO) is encouraged after seeing that the Food and Drug Administration (FDA) will have more cosmetic oversight under the new Omnibus Spending Bill, specifically, the Modernization of Cosmetics Regulation Act of 2022 (MOCRA). The bill will require FDA to increase their oversight of cosmetics and their ingredients, including asbestos. This is a landmark step forward and we are thrilled that the FDA will better regulate asbestos in cosmetics.
Asbestos is a known human carcinogen, and its health risks are well-documented. There is general agreement among U.S. federal agencies, most developed nations, and the World Health Organization (WHO) that there is no established threshold for adverse health effects from asbestos exposure.
Under MoCRA, FDA shall promulgate proposed regulations to establish standardized testing methods for detecting and identifying asbestos in talc-containing cosmetic products not later than one year after the December 29, 2022 and issue final regulations not later than 180 days after the date on which the public comment period on the proposed regulations closes.
In addition, FDA has been conducting testing for asbestos contamination in talc-containing cosmetic products and reporting these results on a regular basis on their Talc | FDA webpage since 2019. Most recently 50 samples were analyzed using polarized light microscopy and transmission electron microscopy for the 2022 Testing of Talc-Containing Cosmetics for Asbestos.
Each year, the U.S. spends $716 billion on cosmetics, according to an HBO Max Docuseries “Not So Pretty”, which spotlights the dangers of asbestos in talc-based cosmetics. In February 2020, ADAO spoke at a public meeting held by the Environmental Protection Agency (EPA) about asbestos testing and the health risk of asbestos exposure.
Also in 2020, ADAO joined colleagues and publicly challenged Johnson & Johnson (J&J) to stop selling its asbestos-contaminated talc-based baby powder in the global market, as it faces multiple lawsuits here in the United States. We joined over 200 organizations from 50 countries who called on J&J again to remove the asbestos-contaminated talc-based powder from consumer shelves. As pointed out by ADAO’s ally, Black Women for Wellness, in the letter to J&J, the powder has been aggressively marketed to women of color for decades, putting Black women at a higher risk of asbestos-caused illness such as ovarian cancer and asbestosis.
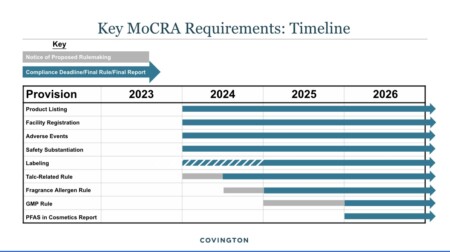
We believe the oversight provided in the Omnibus Spending Bill will help FDA use their regulatory power to reduce and eliminate the risk asbestos in personal care products and cosmetics. EPA has regulatory powers to prohibit commercial asbestos imports and use; however, the current Chrysotile Asbestos Part 1 Rule only bans one fiber and six conditions of use. A landmark step forward, but not a ban and the industry is already posturing to sue the EPA.
That is why ADAO and other stakeholders are working with Congress on The Alan Reinstein Ban Asbestos Now (ARBAN) Act would ban asbestos once and for all. ADAO firmly believes that all people exposed to asbestos deserve their day in court, which is why ARBAN would not impact talc litigation. In very positive news for asbestos court cases, punitive damages decisions for defendants in asbestos litigation matters seem to be trending upwards, meaning people who were exposed to asbestos are receiving the justice they deserve. We believe that the Omnibus Spending Bill and ARBAN will ensure less people are exposed, while still allowing and encouraging those who have been exposed to fight for what they deserve in court.
MoCRA is another landmark step forward in efforts to prevent toxic chemical exposures to protect public health. Each year, over 40,000 Americans die from asbestos-caused illnesses.. We need both regulation and legislation in order to end this man-made disaster. ADAO will continue to support, FDA, EPA, and Congress to protect the public from toxic substances.
Onward,
Linda Reinstein
